The use of green hydrogen in oil refining is an emerging trend aimed at reducing carbon emissions. Green hydrogen is produced by splitting water into hydrogen and oxygen using electricity generated from renewable or nuclear sources. This method of production ensures that green hydrogen is free of greenhouse gas emissions, which is crucial for its role in decarbonizing industries like oil refining.
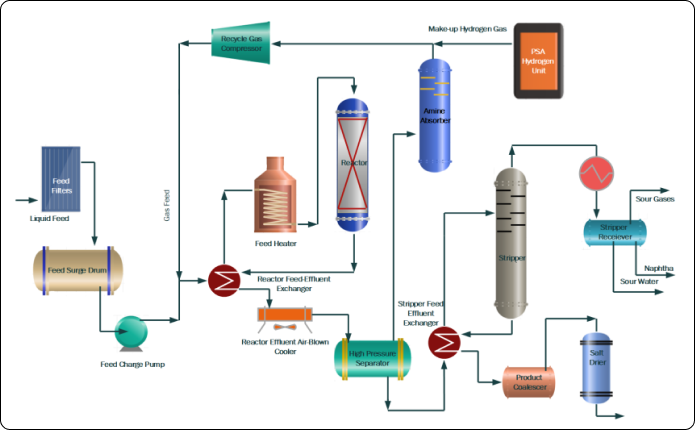
Hydrogen plays a pivotal role in oil refining, primarily in the processes of hydrotreating and hydrocracking. These two processes are critical in refining because they are responsible for consuming over 90% of the hydrogen used in the sector. Hydrotreating is essential for reducing sulfur content in finished petroleum products. Sulfur compounds, if not removed, can lead to harmful emissions when the fuel is burned and can also corrode engines and other machinery. By removing sulfur, hydrotreating ensures that the fuels meet environmental standards and are safer for use in vehicles and machinery.
Hydrocracking, on the other hand, is a process that breaks down heavier fractions of petroleum into lighter, more valuable products like gasoline and jet fuel. This process is particularly important for maximizing the yield of high-value products from crude oil. Hydrocracking involves breaking down larger hydrocarbon molecules into smaller ones, a reaction that requires hydrogen. The process increases the amount of usable fuel derived from crude oil, making it a key step in maximizing the efficiency and profitability of oil refining.
Currently, the majority of the hydrogen used in these processes is sourced as a by-product from other refinery operations, such as catalytic reforming and ethylene cracking. Catalytic reforming involves the conversion of heavy naphthas into high-octane gasoline components, while ethylene cracking is primarily concerned with the production of ethylene, a building block for various petrochemicals. Both of these processes generate hydrogen as a by-product, which is then used in hydrotreating and hydrocracking.
However, there is a growing trend towards replacing this hydrogen with low-carbon alternatives, particularly green hydrogen. This shift is driven by the increasing stringency of environmental regulations and the global commitment to reducing carbon emissions. Green hydrogen, produced through the electrolysis of water using renewable energy sources, offers a much lower carbon footprint compared to conventional hydrogen production methods. The integration of green hydrogen into oil refining can significantly reduce the carbon emissions associated with these processes.

Despite its environmental benefits, green hydrogen production is more expensive than traditional methods due to higher electricity costs and the limited availability of zero-carbon power. This means that for green hydrogen to be more widely adopted in oil refining, there needs to be a decrease in power costs and an increase in the efficiency and availability of zero-carbon power sources.
There are ongoing efforts and projects aimed at integrating green hydrogen into refinery processes. For instance, the REFHYNE project in Germany is working on installing and operating a large electrolyser at a refinery to provide bulk quantities of green hydrogen. This project is a part of a broader move towards decarbonizing the refining process and reducing emissions, a trend that is expected to continue and expand in the 2020s.
The potential market for low-carbon hydrogen in oil refining is significant. By 2050, the demand for low-carbon hydrogen in the global refining sector could reach 50 million tonnes per annum. This transition to green hydrogen could play a crucial role in reducing up to 35% of refining carbon emissions.